DEVELOPMENT AND VALIDATION OF REVERSE PHASE HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY (RP-HPLC) METHOD FOR ESTIMATION OF TRIAMCINOLONE ACETONIDE (KENACORT A-40) IN BULK AND ITS PHARMACEUTICAL FORMULATION
DOI:
https://doi.org/10.71146/kjmr223Keywords:
Method Development, Validation, R.P.- HPLC, Triamcinolone AcetonideAbstract
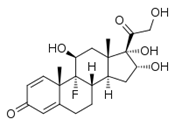
A straightforward and precise Reverse Phase High-Performance Liquid Chromatography (RP-HPLC) for the estimation of triamcinolone Acetonide was developed. Methanol: water mixture was used as the mobile phase. The validation parameters like linearity, accuracy, precision, robustness, the Limit of detection, and the Limit of quantitation were performed for Triamcinolone Acetonide. Concentration was found to be around 25mg/mL. Recovery and assay studies of Ranitidine HCl were 99%, indicating that the proposed method can be adopted for quality control analysis of Ranitidine HCl. Validation of the Method for the drug and its formulation was found to be Precise, Accurate, Selective, and Sensitive. RSD was recorded up to the maximum Limit of 1.302% within the allowed Limit of%. Accuracy/Recovery was seen as 100%, 120%. Intermediate Precision was observed as 100%, while reproducibility was found as 100%. Co-relation Co-efficient & Y-intercept are also within the Limit of 0.9988 & 0.2512 with linear graph.
Downloads
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Nisar Ahmed Katohar, Abdul Rasheed Kaleri, Junaid Ahmed Buriro, Muhammad Kashif Channa, Wahid Bux Jatoi, Asma Kaleri (Author)

This work is licensed under a Creative Commons Attribution 4.0 International License.






