AN INNOVATIVE ECO-FRIENDLY TECHNIQUE TO MEASURE CHROMIUM WITH BENZOHYDRAZIDE
DOI:
https://doi.org/10.71146/kjmr101Keywords:
Chromium, Benzohydrazide, Chelating agent, SurfactantAbstract
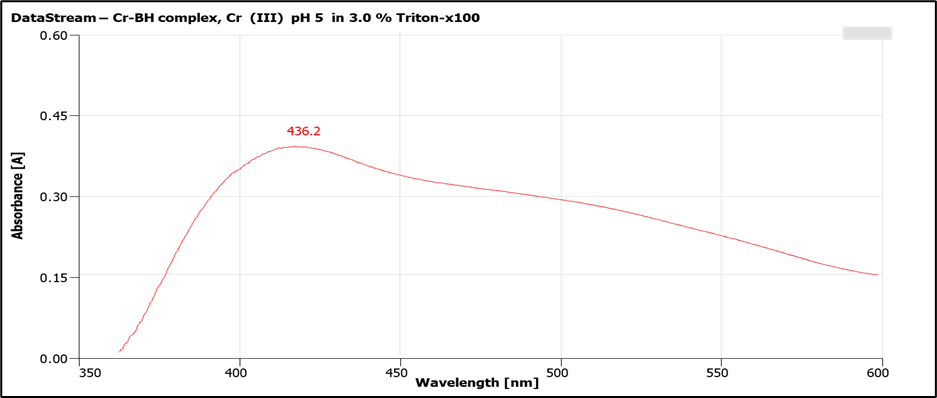
Numerous methodologies exist for trace level analysis of Chromium (Cr) metal ions. Many of these approaches are characterized by their time-consuming nature, complexity, toxicity, and sophistication. Recently, some spectrophotometric methods have been developed that, while less sensitive and selective, offer quicker analyses for determining Cr(III) ions. We innovated suitable, swift, sensitive and selective technique for detecting Cr(III) at trace levels, utilizing Benzohydrazide (BH) as the derivatizing reagent within a 3.0% Triton X-100 surfactant system. This procedure demonstrated improved analytical characteristics. Cr-[BH]3 complex exhibited absorbance maxima at λmax 436.2 nm at pH 5. The method adhered to Lambert-Beer’s law within concentration range of 0.1-4.0 μg/mL, with a 1:3 ligand to metal ratio achieved for the Cr-[BH]3 compound. Sandell's sensitivity was calculated to be 4.9 ng/cm², and the molar absorptivity was determined as ε = 4.03 × 10⁴ L mol⁻¹ cm⁻¹. A detection limit of 4.9 µg/L was successfully established. This method proved highly effective for quantifying Cr(III) ions across diverse samples, including natural, biological, alloy, industrial, and ecological matrices.
Downloads
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Muhammad Kashif Channa, Gul Afshan Soomro, Ghulam Abbas Shar (Author)

This work is licensed under a Creative Commons Attribution 4.0 International License.






